
Капли датского короля
Капли датского короля.
Российская военная фармакопея, 1913 год.


Наше предприятие осуществляет заготовку, сушку и реализацию пряно-ароматического и лекарственного…
Европейская Фармакопея издание 7.0 часть 1 2010 год (Ph. Eur. 7.0) стр. 1145-1146. Чистотел большой
01/2008:1861 corrected 6.0
GREATER CELANDINE
Chelidonii herba
DEFINITION
Dried, whole or cut aerial parts of Chelidonium majus L. collected during flowering.
Content: minimum 0.6 per cent of total alkaloids, expressed as chelidonine (C20H19NO5; Mr 353.4) (dried drug).
IDENTIFICATION
A. The stems are rounded, ribbed, yellowish or greenish-brown, somewhat pubescent, about 3-7 mm in diameter, hollow and mostly collapsed. The leaves are thin, irregularly pinnate, the leaflets ovate to oblong with coarsely dentate margins, the terminal leaflet often three-lobed; the adaxial surface is bluish-green and glabrous, the abaxial surface paler and pubescent, especially on the veins. The flowers have 2 deeply concavo-convex sepals, readily removed, and 4 yellow, broadly ovate, spreading petals about 8-10 mm long; the stamens are numerous, yellow, and a short style arises from a superior ovary; long, capsular, immature fruits are rarely present.
B. Reduce to a powder (355) (2.9.12). The powder is dark greyish-green or brownish-green. Examine under a microscope using chloral hydrate solution R. The powder shows the following diagnostic characters: numerous fragments of leaves in surface view, the epidermal cells with sinuous walls; anomocytic stomata (2.8.3) occur on the abaxial surface only; covering trichomes long, uniseriate, with thin walls and usually fragmented; vascular tissue from the leaves and stems with groups of fibres, pitted and spirally thickened vessels and associated latex tubes with yellowish-brown contents; occasional fragments of the corolla with thin-walled, partly papillose cells containing numerous pale yellow droplets of oil; spherical pollen grains about 30-40 pm in diameter with 3 pores and a finely pitted exine.
C. Thin-layer chromatography (2.2.27).
Test solution. To 0.4 g of the powdered drug (710) (2.9.12) add 50 mL of dilute acetic acid R. Boil in a water-bath under a reflux condenser for 30 min. Cool and filter. To the filtrate add concentrated ammonia R until a strong alkaline reaction is produced. Shake with 30 mL of methylene chloride R. Dry the organic layer over anhydrous sodium sulfate R, filter and evaporate in vacuo to dryness. Dissolve the residue in 1.0 mL of methanol R.
Reference solution. Dissolve 2 mg of papaverine hydrochloride R and 2 mg of methyl red R in 10 mL of ethanol (96 per cent) R.
Plate: TLC silica gel plate R.
Mobile phase: anhydrous formic acid R, water R, propanol R (1:9:90 V/V/V).
Application: 10 μL, as bands.
Development: over a path of 10 cm.
Drying: in air.
Detection: spray with potassium iodobismuthate solution R and dry in air; spray with sodium nitrite solution R and allow to dry in air; examine in daylight.
Results: see below the sequence of the zones present in the chromatograms obtained with the reference solution and the test solution. Furthermore, other weaker zones may be present in the chromatogram obtained with the test solution. 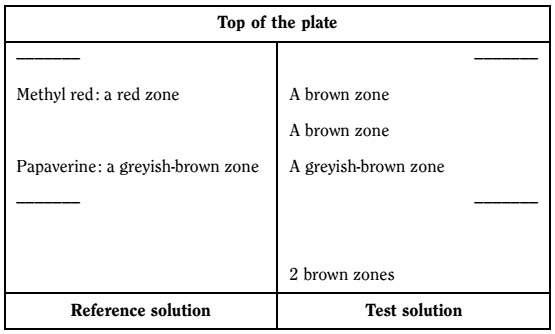
TESTS
Foreign matter (2.8.2): maximum 10.0 per cent.
Loss on drying (2.2.32): maximum 10.0 per cent, determined on 1.000 g of the powdered drug (355) (2.9.12) by drying in an oven at 105 °C for 2 h.
Total ash (2.4.16): maximum 13.0 per cent.
ASSAY
Test solution. To 0.750 g of the powdered drug (710) (2.9.12), add 200 mL of dilute acetic acid R and heat on a water-bath for 30 min, shaking frequently. Cool and dilute to 250.0 mL with dilute acetic acid R. Filter. Discard the first 20 mL of the filtrate. To 30.0 mL of the filtrate add 6.0 mL of concentrated ammonia R and 100.0 mL of methylene chloride R. Shake for 30 min. Separate the organic layer, place 50.0 mL in a 100 mL round-bottomed flask and evaporate to dryness in vacuo at a temperature not exceeding 40 °C. Dissolve the residue in about 2-3 mL of ethanol (96 per cent) R, warming slightly. Transfer the solution to a 25 mL volumetric flask by rinsing the round-bottomed flask with dilute sulfuric acid R and dilute to 25.0 mL with the same solvent. To 5.0 mL of the solution, add 5.0 mL of a 10 g/L solution of chromotropic acid, sodium salt R in sulfuric acid R in a 25 mL volumetric flask, stopper the flask and mix carefully. Dilute to 25.0 mL with sulfuric acid R and stopper the flask.
Compensation liquid. Prepare at the same time and in the same manner as for the test solution: place in a 25 mL volumetric flask 5.0 mL of dilute sulfuric acid R and 5.0 mL of a 10 g/L solution of chromotropic acid, sodium salt R in sulfuric acid R, stopper the flask and mix carefully. Dilute to 25.0 mL with sulfuric acid R and stopper the flask.
Place both solutions on a water-bath for 10 min. Cool to about 20 °C and dilute if necessary to 25.0 mL with sulfuric acid R. Measure the absorbance (2.2.25) of the test solution at 570 nm by comparison with the compensation liquid.
Calculate the percentage content of total alkaloids, expressed as chelidonine, using the following expression: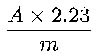
i.e. taking the specific absorbance of chelidonine to be 933.
A = absorbance at 570 nm,
m = mass of the substance to be examined, in grams.