
Капли датского короля
Капли датского короля.
Российская военная фармакопея, 1913 год.


Наше предприятие осуществляет заготовку, сушку и реализацию пряно-ароматического и лекарственного…
Европейская Фармакопея издание 7.0 часть 1 2010 год (Ph. Eur. 7.0) стр.1052-1053 Аниса плоды
07/2010:0262
ANISEED
Anisi fructus
DEFINITION
Whole dry cremocarp of Pimpinella anisum L.
Content: minimum 20 mL/kg of essential oil (anhydrous drug).
CHARACTERS
Reminiscent odour of anethole.
The fruit is a cremocarp and generally entire; a small fragment of the thin, rigid, slightly curved pedicel is frequently attached.
IDENTIFICATION
A. The cremocarp is ovoid or pyriform and slightly compressed laterally, yellowish-green or greenish-grey, 3-5 mm long and up to 3 mm wide, surmounted by a stylopod with 2 short, reflexed stylar points. The mericarps are attached by their tops to the carpophore with a plane commissural surface and a convex dorsal surface, the latter being covered with short, warty trichomes visible using a lens; each mericarp shows 5 primary ridges, running longitudinally, comprising 3 dorsal ridges and 2 lateral ridges, non-prominent, and lighter in colour.
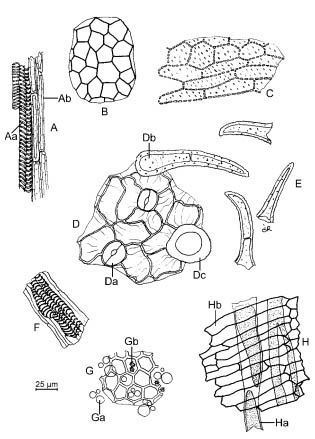
A. Xylem vessels (Aa) and fibres (Ab) from the pedicel
B. Testa
C. Sclereids from the mesocarp
D. Epicarp with striated cuticle and anomocytic stomata (Da), entire covering trichome (Db) and base of covering trichome (Dc)
E. Broken covering trichomes
F. Xylem vessels
G. Fragment of endosperm containing oil droplets (Ga) and calcium oxalate cluster crystals (Gb)
H. Fragment of vitta (Ha) accompanied by oblong thin-walled cells from the endocarp (Hb)
Figure 0262.-1. - Illustration of powdered herbal drug of aniseed (see Identification B)
B. Reduce to a powder (355) (2.9.12). The powder is greenish-yellow or brownish-green. Examine under a microscope using chloral hydrate solution R. The powder shows the following diagnostic characters: whole or broken covering trichomes, mostly unicellular, sometimes curved, with blunt apex and warty cuticle; fragments of epicarp with striated cuticle, occasional anomocytic stomata (2.8.3); fragments of numerous narrow, branched vittae, often accompanied by fragments of endocarp with thin-walled, elongated cells; fragments of testa consisting of a layer of brown, polyhedral cells, with thin walls; fragments of endosperm containing aleurone grains and calcium oxalate cluster crystals; square or oblong sclereids from the mesocarp or the commissural zone of the fruit and bundles of short sclerenchymatous fibres from the carpophore and the pedicel, accompanied by vessels with spiral or annular thickening.
C. Thin-layer chromatography (2.2.27).
Test solution. Shake 0.10 g of the powdered drug (1400) (2.9.12) with 2mL of methylene chloride R for 15 min. Filter and carefully evaporate the filtrate to dryness on a water-bath at 60 °C. Dissolve the residue in 0.5 mL of toluene R.
Reference solution. Dissolve 3 µL of anethole R and 40 µL of olive oil R in 1 mL of toluene R.
Plate: TLC silica gel GF254 plate R.
Mobile phase: toluene R.
Application: 2 µL and 3 µL of the test solution, then 1 µL, 2 µL and 3 µL of the reference solution, at 2 cm intervals.
Development: over a path of 10 cm.
Drying: in air.
Detection A: examine in ultraviolet light at 254 nm.
Results A: the chromatograms show a quenching zone (anethole) in the central part against a light background.
Detection B: spray with a freshly prepared 200 g/L solution of phosphomolybdic acid R in ethanol (96 per cent) R, using 10 mL for a 200 mm square plate, and heat at 120 °C for 5 min.
Results B: the spots due to anethole appear blue against a yellow background. In the chromatogram obtained with 2 µL of the test solution, the spot due to anethole is intermediate in size between the corresponding spots in the chromatograms obtained with 1 µL and 3 µL of the reference solution. The chromatograms obtained with the test solution show in the lower third a blue spot (triglycerides) similar in position to the spot in the lower third of the chromatograms obtained with the reference solution (triglycerides of olive oil).
TESTS
Water (2.2.13): maximum 70 mL/kg, determined on 20.0 g of the powdered drug.
Total ash (2.4.16): maximum 12.0 per cent.
Ash insoluble in hydrochloric acid (2.8.1): maximum 2.5 per cent.
ASSAY
Carry out the determination of essential oils in herbal drugs (2.8.12). Use 10.0 g of the drug reduced to a coarse powder immediately before determination, a 250 mL round-bottomed flask, and 100 mL of water R as the distillation liquid. Place 0.50 mL of xylene R in the graduated tube. Distil at a rate of 2.5-3.5 mL/min for 2 h.